View resources
- Emergency management flowchart [PDF 551 KB] - 1 page
- Nomogram [PDF 331 KB] -1 page
- N-Acetylcysteine order form - Child (less than 20kg) [PDF 664 KB] - 1 page
- N-Acetylcysteine order form - Child (20-50kg) [PDF 236 KB] - 1 page
- N-Acetylcysteine order form - Child (greater than 50kg) [PDF 668 KB ] - 1 page
Key points
- Toxic paracetamol ingestions require prompt treatment with acetylcysteine infusion to avoid serious hepatic injury and death.
- The need for acetylcysteine is guided by measuring a serum paracetamol concentration and plotting this on the paracetamol treatment nomogram.
- Overdoses involving modified release paracetamol and liquid paracetamol require different management.
- Administer acetylcysteine immediately if paracetamol concentration levels are not likely to be available within eight hours of a potentially toxic ingestion (due to delay in presentation to ED or time for testing or uncertain time of ingestion) or patient has symptoms of hepatic injury.
- Careful attention is required to avoid acetylcysteine dosing errors. Fluid adjustment is required for children less than 50kg.
- Seek urgent toxicological advice from Poisons Information (Ph: 131126) for very large overdoses (50 g or 1 g/kg), IV paracetamol overdoses or if evidence of hepatotoxicity (ALT greater than 1,000 U/L). Critical care may be required.
Purpose
This document provides clinical guidance for all staff involved in the care and management of a child presenting to an Emergency Department (ED) in Queensland following a paracetamol ingestion.
This guideline has been developed by senior ED clinicians and Paediatricians across Queensland, with input from Queensland Poisons Information Centre, Clinical Toxicology; Princess Alexandra Hospital and Pharmacy, Gastroenterology and PICU; Queensland Children’s Hospital, Brisbane. It has been endorsed for use statewide by the Queensland Emergency Care of Children Working Group in partnership with the Queensland Emergency Department Strategic Advisory Panel and the Healthcare Improvement Unit, Clinical Excellence Queensland.
Introduction
Paracetamol is a widely used analgesic that is readily available in many different preparations. Accidental or deliberate overdose can cause hepatic failure and death. This can be prevented by the early administration of acetylcysteine.1
This guideline is based on the recommendations made in 2020 by a group of Australasian Clinical Toxicologists consulting to the Poisons Information Centre.1
While there are certain groups who are at higher risk of hepatotoxicity (such as those with malnutrition, eating disorders, cystic fibrosis or acute viral infections) the recommended management is conservative and so remains unchanged.
Pharmacokinetics
Paracetamol is rapidly absorbed in the small intestine and reaches peak concentrations within 30 minutes for liquid preparations and one to two hours for standard tablet preparations. Distribution then occurs within two hours for liquid preparations and four hours for standard tablet preparations.2 Hepatic biotransformation results in 90% of paracetamol being metabolised to inactive sulphate and glucuronide conjugates which are then excreted by the kidneys. The remaining 10% requires cytochrome P450 to make an intermediary compound of N-acetyl-p-benzoquinone imine (NAPQI) which then in turn binds to intracellular glutathione for renal excretion. Depletion of glutathione occurs with higher production of NAPQI which subsequently binds to other proteins and thus damages hepatocytes. Clinical or biochemical evidence of this damage may take up to 24 hours post overdose to become apparent.3
Acetylcysteine is an effective antidote to paracetamol toxicity by increasing the synthesis and availability of glutathione and directly binding to NAPQI. Appropriate treatment commencing within eight hours of the overdose will prevent almost all serious hepatic injury. 4
Assessment
The aim of the initial assessment is to determine the risk of hepatic injury following paracetamol ingestion.
History
History-taking should include information on:
- number, quantity, concentration* and timing of ingestions
- preparation – immediate or modified release, liquid
- intentional or accidental
- symptoms of hepatic injury (such as abdominal pain, nausea or vomiting, anorexia)
* at least 4 different concentrations of liquid paracetamol in Australia
Examination
Full examination focussing on eliciting any toxidromes to suggest co-ingestion, neurological status for co-ingestion risk and hepatic encephalopathy, and serial abdominal examinations which can elicit right upper quadrant tenderness.
Calculation of ingested dose
Use the available information to calculate the dose(mg) per kilogram of paracetamol ingested. When in doubt of the quantity, use the maximum possible ingested dose to determine the potential for hepatic injury.
| Age | Acute single ingestion | Repeated supratherapeutic ingestion |
|---|---|---|
| 0 – 6 years | ≥ 200 mg/kg immediate release |
Any of the following:
|
| Over 6 years | ≥ 200 mg/kg or 10g immediate release (whichever is lower) |
Any of the following:
|
| All ages | Any modified release ingestion exceeding the daily therapeutic dose | Any modified release ingestion exceeding the daily therapeutic dose. Even ingestion of doses < 200mg/kg or ≤ 10g are potentially toxic. |
*Use the ideal body weight for body weight calculations in obese children.
If the patient has taken a mixture of immediate release and modified release paracetamol, treat as a modified release ingestion, and contact Queensland Poisons information Centre (QPIC) (Ph: 131126) or local toxicology service. 4
Investigations
Administer acetylcysteine immediately if paracetamol concentration levels are not likely to be available within eight hours of a potentially toxic ingestion (due to delay in presentation to ED or time for testing or uncertain time of ingestion) or patient has symptoms of hepatic injury (abdominal pain, nausea, vomiting and anorexia). Do not delay for paracetamol concentration levels.
Serum paracetamol concentration testing is used to determine the need for acetylcysteine (by plotting on the treatment nomogram provided below). Testing is recommended for patients with a history of:
- ingesting a toxic dose (refer to table in Assessment section)
- deliberate self‐poisoning regardless of the stated ingested dose
- accidental exposures if uncertain of ingested dose
| Investigations recommended for the management of paracetamol overdose | |||
|---|---|---|---|
| Children aged less than 6 years post-ingestion of liquid paracetamol | Serum paracetamol concentration at 2 hours post-ingestion. Concentrations less than 150 mg/L require no further treatment. Repeat at 4 hours post-ingestion if initial concentration is greater than or equal to 150 mg/L. If patient presents after 4 hours, or is over 6 years of age, manage as below. | ||
| Patients with single potentially toxic immediate release paracetamol ingestion | Time of presentation | Testing | |
| Within 8 hours of ingestion | Serum paracetamol concentration and ALT within 4-8 hours post-ingestion. If treatment indicated, repeat paracetamol concentration and ALT two hours before the end of the second bag of acetylcysteine infusion. | ||
| 8-24 hours post-ingestion | Serum paracetamol concentration and ALT on presentation and commence acetylcysteine while awaiting level. If ongoing treatment indicated, repeat paracetamol concentration and ALT 2 hours before the end of the second bag of acetylcysteine infusion. | ||
| Greater than 24 hours post-ingestion | Serum paracetamol concentration, transaminases (ALT/AST), INR/PT, creatinine, urea, glucose and arterial or venous blood gas on presentation and commence acetylcysteine while awaiting level3. Follow up testing as clinically indicated. | ||
| Unknown time of ingestion | Serum paracetamol concentration, ALT and INR on presentation and commence acetylcysteine while awaiting level. Seek advice from Poisons Information (Ph: 131126) for further testing. | ||
| Patients post-ingestion of modified release paracetamol (e.g. Panadol Osteo®, Osteomol®) |
Serum paracetamol concentration minimum of 4 hours post-ingestion. Repeat serum paracetamol concentration 4 hours after initial testing to capture the delayed release. If treatment indicated, start acetylcysteine. Repeat paracetamol concentration and ALT 2 hours before the end of the second bag of acetylcysteine infusion to guide need for ongoing decontamination and/or treatment. | ||
| Patients with potentially toxic repeated supratherapeutic ingestions | Serum paracetamol concentration and ALT on presentation. Repeat at eight hours after initial measurement if either serum paracetamol concentration greater than 20 mg/L, or ALT greater than 50 U/L or increasing and if treatment indicated, start acetylcysteine. Check ALT every 12 hours if ALT greater than 50 U/L or the repeat paracetamol concentration is greater than 10 mg/L. | ||
| Patients with multiple or staggered ingestion with intent of self harm | If multiple or staggered ingestion (over more than 2 hours) with intent of self harm, treat as per acute immediate release ingestion with serum paracetamol level and ALT within 4-8 hours from the time of first dose. If the most recent ingestion occurs within 2 hours of initial bloods, repeat paracetamol concentration 2 hours later to capture ongoing absorption. | ||
Rumack-Mathew paracetamol treatment nomogram
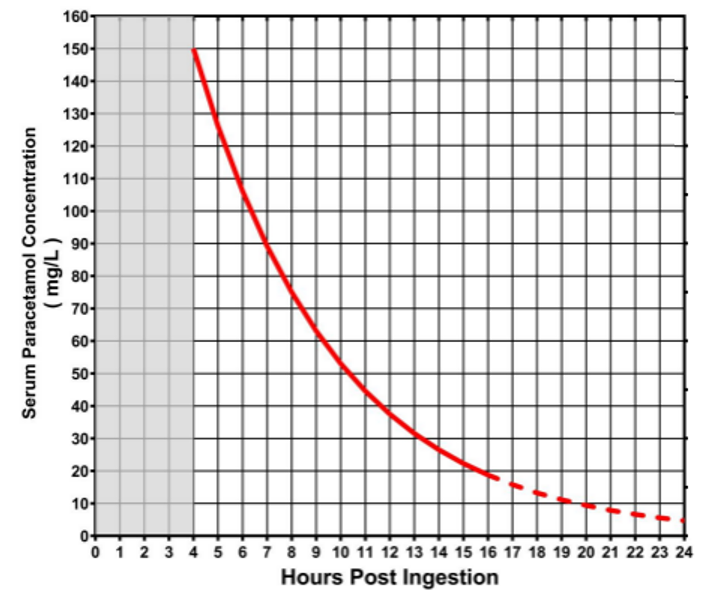
Reproduced from Daly FF et al. Guidelines for the management of paracetamol poisoning in Australia and New Zealand-explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Med. J. Aust. 2008;188 (5): 296-301 with permission from John Wiley and Sons. Link to article. © 2008 AMPCo Pty Ltd. All rights reserved.
Management
Refer to flowchart [PDF 997.58 KB] for a summary of the emergency management for a child following a paracetamol ingestion:
Contact a Clinical Toxicologist via Queensland Poisons information Centre (QPIC) (ph: 131 126) or local toxicology service urgently for:
- overdoses of 50 g or 1 g/kg (always use lower threshold)
- measured paracetamol concentration is double the nomogram line
- IV paracetamol errors or overdoses
- evidence of hepatotoxicity (ALT greater than 1,000 U/L)
Higher concentrations of acetylcysteine may be required. Contact paediatric critical care specialist (onsite or via Retrieval Services Queensland (RSQ)) as advised by Poisons/QPIC/Clinical Toxicologist.
Refer patients with a deliberate overdose for a psychiatric assessment as per local practices.
Decontamination - Activated charcoal
Activated charcoal may prevent or reduce the need for treatment with acetylcysteine if used appropriately.
Activated charcoal (1g/kg) is only routinely recommended for cooperative patients aged greater than six years if able to be administered up to 2 hours post-ingestion for potentially toxic overdoses of immediate release paracetamol. It may be given up to 4 hours post-ingestion for modified release paracetamol preparations, or for very large overdoses of immediate release paracetamol (≥ 500 mg/kg or ≥ 30g). Multiple doses of activated charcoal may be given for very large overdoses of modified release paracetamol on advice from Poisons Information Centre/Clinical Toxicologist. Activated Charcoal is not recommended in liquid preparation overdose due to the fast absorption time.
Acetylcysteine
Acetylcysteine following single toxic immediate release paracetamol ingestion
The need for acetylcysteine is guided by serum paracetamol concentration plotted on the paracetamol treatment nomogram (see Assessment section).
Additional management is required for modified release paracetamol ingestions. Refer to section below. Treat mixed immediate and modified release ingestions as modified and contact Queensland Poisons information Centre (QPIC) (Ph: 131 126) or local toxicology service.
ACETYLCYSTEINE following single toxic immediate release paracetamol ingestion
| Time from ingestion | Indications for acetylcysteine | |
|---|---|---|
  | 2 hours | Acetylcysteine will not be required for well children aged less than 6 years with serum paracetamol concentration less than 150 mg/L at 2 hours post-ingestion of liquid paracetamol. If greater than or equal to 150 mg/L do not commence acetylcysteine but repeat level at 4 hours and manage as below. |
| 4-8 hours | Commence acetylcysteine if:
If initial serum paracetamol concentration is greater than double the nomogram line, patients will require a double dose second bag of acetylcysteine (see “Acetylcysteine administration” below). Recommend discussion with Poisons Information Centre (Ph: 131 126). Await serum levels if results are expected within 8 hours of ingestion. | |
| 8-24 hours | Commence acetylcysteine immediately after taking bloods (see Investigations). Continue acetylcysteine if serum paracetamol concentration above the nomogram treatment line or ALT greater than 50 U/L. If initial serum paracetamol concentration is greater than double the nomogram line, patients will require a double dose second bag of acetylcysteine (see “Acetylcysteine administration” below). Recommend discussion with Poisons Information Centre (Ph: 131 126). | |
| ≥ 24 hours or unknown | Commence acetylcysteine immediately after taking bloods (see Investigations). Continue acetylcysteine if paracetamol concentration is greater than 10 mg/L or ALT greater than 50 U/L. If serum paracetamol concentration is higher than 100 mg/L, seek advice from Poisons Information Centre clinical toxicologist (Ph: 131 126) or local toxicology service. | |
Acetylcysteine following staggered toxic ingestion of immediate release paracetamol with intent of self harm
Treatment is as per acute immediate release ingestion, using the time of first ingestion.
If a dose was ingested within 2 hours of initial bloods, repeat paracetamol concentration 2 hours later to capture ongoing absorption and commence acetylcysteine if either level above nomogram treatment line (see Investigations).
Acetylcysteine following toxic modified release paracetamol ingestions
Modified release paracetamol preparations (such as Panadol Osteo® and Osteomol® both with 665mg paracetamol/tablet) result in potentially delayed peak concentrations above the nomogram treatment line. A single measurement of paracetamol level is not adequate to make decisions around acetylcysteine administration if an unknown quantity or a potentially toxic quantity has been ingested.
| Acetylcysteine administration following modified release paracetamol ingestions | |
|---|---|
| Modified release ingestion ≤ 200 mg/kg or ≤ 10g |
|
| Modified release ingestion ≥ 200 mg/kg or ≥ 10g or unknown |
If either of the paracetamol levels are double the nomogram treatment line, administer double dose second bag of acetylcysteine (see “Acetylcysteine administration”). Recommend discussion with Poisons Information Centre (Ph: 131 126) or local toxicology service. |
| Modified release massive ingestion ≥ 500 mg/kg or ≥ 30 g | Administer a double dose second bag of acetylcysteine (see “Acetylcysteine administration”). Recommend discussion with Poisons Information Centre (Ph: 131 126) or local toxicology service. |
Acetylcysteine following repeated supratherapeutic ingestions
Acetylcysteine administration following repeated supratherapeutic ingestions*
Commence acetylcysteine if serum paracetamol concentration taken on presentation is greater than 20 mg/L or ALT greater than 50 U/L.
If acetylcysteine is indicated, repeat levels at 8 hours after initial testing. Discontinue acetylcysteine if ALT is less than 50 U/L or static, AND paracetamol concentration is less than 10 mg/L. Otherwise continue acetylcysteine, recheck ALT every twelve hours and seek advice from Queensland Poisons information Centre (QPIC) (Ph: 131 126) or local toxicology service.
*Refer to Assessment section for definition
Acetylcysteine administration
Careful attention is required when ordering fluids. Fluid adjustment orders are required for smaller children due to risk of hyponatremia if using the total adult fluid volume (1500mls). Secondary seizures have resulted when using 5% glucose. 4,5,6
Refer to the Acetylcysteine guideline and use the appropriate order form (based on child’s weight) or the electronic ordering system.
The total acetylcysteine dosing is 300 mg/kg administered over 20 hours in two sequential IV infusions in crystalloid solution (200 mg/kg over 4h, then 100 mg/kg over 16h). Double the dose of the second bag of acetylcysteine (to 200mg/kg over 16h) for those patients with doses resulting in high paracetamol concentrations more than double the nomogram line, or those who ingest ≥ 30 g or ≥ 500 mg/kg of modified release paracetamol. Dosing is calculated on actual body weight up to 110 kg (with dosing based on 110 kg weight for children over 110 kg). Acetylcysteine is packaged in 10 mL ampoules each containing 2,000 mg (20%). Doses are written in mg.
Where a two bag regime is available on smart pumps, use these profiles. If not available, confirm if the general acetylcysteine profile will allow the infusion to run. If not, revert to the mL/hour functionality (if programmed). Due to differences in doses and durations, it is not suitable to use the superseded three bag regime profile to run the two bag regime.
Prescribe the entire treatment course at the time of the initial presentation to avoid administration delays.
Ceasing acetylcysteine infusion for single ingestions of immediate or modified release paracetamol
- Measure paracetamol concentration and ALT two hours before the end of the second bag of acetylcysteine infusion.
- If paracetamol concentration is less than 10 mg/L and ALT less than 50 U/L, no further treatment required after infusion is complete.
- If paracetamol concentration is greater than 10 mg/L, or ALT is greater than 50 U/L, continue the acetylcysteine infusion and seek advice from Poisons Information Centre (Ph: 131 126).
Ceasing acetylcysteine infusion for repeated supratherapeutic paracetamol ingestion
- Repeat paracetamol concentration 8 hours after initial blood tests
- If paracetamol concentration is less than 10 mg/L and ALT less than 50 U/L, cease acetylcysteine
- If paracetamol concentration is greater than 10 mg/L, or ALT is greater than 50 U/L, continue the acetylcysteine infusion and seek advice from Poisons Information Centre (Ph: 131 126).
Adverse drug reactions
Anaphylactoid reactions including rash, pruritus, angioedema, bronchospasm and rarely hypotension may occur following acetylcysteine administration with females and asthmatics at higher risk. Progression to a more clinically significant reaction is rare.
If drug reactions occur, slow the infusion or temporarily cease the infusion, treat with antihistamines or bronchodilators and restart once the reaction settles.
Ongoing liver impairment
Seek specialist advice (Toxicology/Gastroenterology/Critical Care) for patients with ongoing evidence of liver impairment.
For patients with ongoing liver impairment, continue acetylcysteine and 12-hourly-blood-testing until clinically improving, ALT is reducing, INR is improving and less than 2.0 and the paracetamol level is less than 10mg/L.
Indications for referral to a liver transplant unit
- INR greater than 3.0 at 48 hours or greater than 4.5 at any time
- oliguria or creatinine greater than 200 µmol/L
- persistent acidosis pH less than 7.3 or lactate >3 mmol/L
- systolic hypotension despite resuscitation
- hypoglycaemia
- severe thrombocytopenia
- encephalopathy not otherwise explained 1
Escalation and advice outside of ED
Clinicians can contact the services below if escalation of care outside of senior clinicians within the ED is needed, as per local practices. Transfer is recommended if the child requires a higher level of care.
Critical care is unlikely to be required following a paracetamol overdose in isolation but may be required if co-ingestions have occurred. Seek critical care advice (onsite or via RSQ) if advised by toxicologist or child is critically unwell.
| Toxicology advice is required for the following: |
|---|
|
| Reason for contact | Who to contact |
|---|---|
| Advice (including management, disposition or follow-up of all children requiring a ACETYLCYSTEINE infusion) |
Follow local practices. Options:
|
| Referral | First point of call is the onsite/local paediatric service |
Inter-hospital transfers
| Do I need a critical transfer? |
|
| Request a non-critical inter-hospital transfer |
|
| Non-critical transfer forms |
|
Disposition
When to consider discharge from ED
Consider discharge for the following patients:
- Acetylcysteine infusion not required (based on assessment of serum paracetamol concentration levels or clear history of quantity of accidental ingestion)
AND
- if ingestion deliberate, a psychiatric assessment has been conducted as appropriate.
On discharge, educate the family regarding safe paracetamol administration and storage.
Follow-up
Not routinely required.
When to consider admission
Admission to an inpatient service or SSU is recommended for patients requiring ongoing acetylcysteine infusion once serum paracetamol concentration and ALT levels are available.
Related documents
Guidelines
Forms
-
- Chiew AL, Reith D, Pomerleau A, Wong A, Isoardi KZ, Soderstrom J, Buckley NA. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust. 2020 Mar;212(4):175-183. doi: 10.5694/mja2.50428. Epub 2019 Dec 1. PMID: 31786822.
- Chiew AL, Fountain JS, Graudins A et-al. Summary statement: new guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med. J. Aust. 2015;203 (5): 215-8.
- Daly, F.F.S., Fountain, J.S., Murray, L., Graudins, A. and Buckley, N.A. (2008), Guidelines for the management of paracetamol poisoning in Australia and New Zealand – explanation and elaboration. Medical Journal of Australia, 188: 296-302. https://doi.org.10.5694/j.1326-5377.2008.tb01625.x
- Paracetamol poisoning: immediate-release preparations [published 2020 Aug]. In: Therapeutic Guidelines. Melbourne: Therapeutic Guidelines Limited; accessed {Dec 2023}. http://www.tg.org.au
- Marzullo L. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Curr. Opin. Pediatr. 2005;17 (2): 239-45.
- Sung L, Simons JA, Ayneka NL. Dilution of Intravenous N-Acetylcysteine as a Cause of Hyponatremia. Pediatr. 1997;100(3):389-91.
- Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2006; (2): CD003328.
- Furmaga J, Wax P, Kleinschmidt K. N-Acetylcysteine (ACETYLCYSTEINE)-Induced Hyponatremia Caused by an Electronic Medical Record (EMR) Order Error. J Med Toxicol. 2015;11 (3): 355-8.
-
Document ID: CHQ-GDL-60018
Version number: 6.0
Supersedes: 5.0
Approval date: 04/04/2024
Effective date: 19/04/2024
Review date: 04/04/2028
Executive sponsor: Executive Director Medical Services
Author/custodian: Queensland Emergency Care Children Working Group
Applicable to: Queensland Health medical and nursing staff
Document source: Internal (QHEPS) + External
Authorisation: Executive Director Clinical Services
Keywords: Paracetamol, overdose, ingestion, acetylcysteine, paediatric, emergency, guideline, children, 60018
Accreditation references: NSQHS Standards (1-8): 1 , 4, 8
-
This guideline is intended as a guide and provided for information purposes only. View full disclaimer.
Last updated: April 2024