View resources
Key points
- Head injuries are a common ED presentation in children; most are minor.
- Identifying the small proportion with a significant intracranial injury can be challenging.
- CT scan is the gold standard investigation to identify significant intracranial injuries in the acute setting but carries radiation, and in some children, sedation risks.
- Clinical Decision Rules have been developed to guide imaging decisions; PECARN and CHALICE are the most well known.
- A period of neurological observation may be an alternative to immediate CT scan in some children. Some low risk children can be safely discharged without imaging or observation providing other discharge criteria are met.
- Thorough advice should be provided on discharge irrespective of head injury severity or imaging undertaken.
- Urgent paediatric critical care/neurosurgical advice (onsite or via RSQ) should be sought in the deteriorating child or a child with suspected raised ICP.
- Alternative diagnoses and special circumstances such as non-accidental injury should be considered.
Purpose
This document provides clinical guidance for all staff involved in the care and management of a child presenting to an Emergency Department (ED) in Queensland with a head injury.
This guideline has been developed by senior ED clinicians and Paediatricians across Queensland, with input from Neurosurgery and Pharmacy, Queensland Children’s Hospital, Brisbane. It has been endorsed for statewide use by the Queensland Emergency Care of Children Working Group in partnership with the Queensland Emergency Department Strategic Advisory Panel and the Healthcare Improvement Unit, Clinical Excellence Queensland.
Introduction
Head injuries are a common paediatric ED presentation, accounting for 1 – 2% of all presentations to specialist children’s emergency services within Australia.1 Although most are minor, head injuries remain a significant cause of morbidity and mortality.
Children sustaining head injuries at the more severe end of the head injury spectrum are usually readily identifiable and this should prompt immediate (and concurrent) intervention, investigation and referral for definitive management.
Children with clinical features of head injury at the “milder”, and by far more prevalent end of the spectrum, present their own challenges and differentiating the child with the truly low risk head injury from those at risk of a clinically significant injury, such as an intracranial bleed or a depressed skull fracture, can be problematic. While a CT (Computed Tomography) scan is the investigation of choice to exclude such injuries in the acute setting, it is neither feasible nor ethical to scan every child presenting given concerns with radiation exposure, the potential need for sedation and/or transfer, and resource costs.
Several clinical decision rules (CDRs) have been derived to risk stratify children with isolated head injuries and thus to guide clinicians in imaging and discharge decisions. This guideline is informed by the highest performing CDRs in our setting and adopts the concept of the clinico-radiological rule to enable an evidence-based approach to the decision making around head injury management in Queensland.
Clinical decision rules
Clinical decision rules (CDRs) in paediatric head injury have been derived to guide imaging decisions. The most well known and highest quality CDRs are PECARN2 (US study, 33,785 children), CHALICE3 (UK, 22,772) and CATCH4 (Canada, 3688). PECARN and CHALICE CDRs are outlined below. PECARN is designed for children with a GCS >13, offers different rules for those less than two years and those greater than two years and is a clinico-radiological rule with the option to observe or image children at intermediate risk. CHALICE considers all children presenting with a head injury and CATCH was designed for a higher risk cohort. Both PECARN and CHALICE have a negative predictive value of 99.9%, meaning that children who are negative for the rule i.e. low risk, are estimated to have less than 0.1% risk of significant intracranial injury.
A recent Australia New Zealand observational multicentre study, APHIRST,5 examined the performance of all three of these CDRs in our context. All three rules performed well, PECARN had the highest sensitivity, and all three rules had a negative predictive value over 99% (PECARN – 100% (95%CIs 99.9-100; 99.8-100 for each age group); CHALICE 99.8% (95%CI 99.7-99.9). It is important to recognise though, that strict application of the rules would likely have resulted in a much higher imaging rate than currently exists in the 10 tertiary and large mixed hospitals that were part of the study. The APHIRST baseline imaging rate among all children was 10.5%; PECARN if strictly applied could result in an imaging rate as high as 46.6% (depending on whether intermediate risk children were observed or scanned), CHALICE 22% and CATCH 30%. Furthermore, in the APHIRST study, clinician gestalt in children with milder head injuries (GCS 13-15) was found to perform better than any rule (no missed injuries).6 The ten APHIRST sites are either tertiary children’s hospitals or large mixed centres with a strong paediatric focus, and clinician decisions are likely informed by factors including awareness of the CDRs, clinician experience, and the use of observation instead of immediate CT scan as a management option in some cases.
There is considerable overlap between the CDRs in clinical assessment variables (history, mechanism of injury, examination), albeit with some discrepancy over exact details e.g. height of fall, number of vomits and length of any loss of consciousness. These features and the CDRs have been used to inform the statewide guideline. Scope has also been given to allow for observation as an initial option in some children deemed at intermediate risk.
The Paediatric Emergency Care Applied Research Network (PECARN) suggested CT algorithm for children younger than 2 years (A) and for those aged 2 years and older (B) with GCS scores of 14-15 after head trauma*
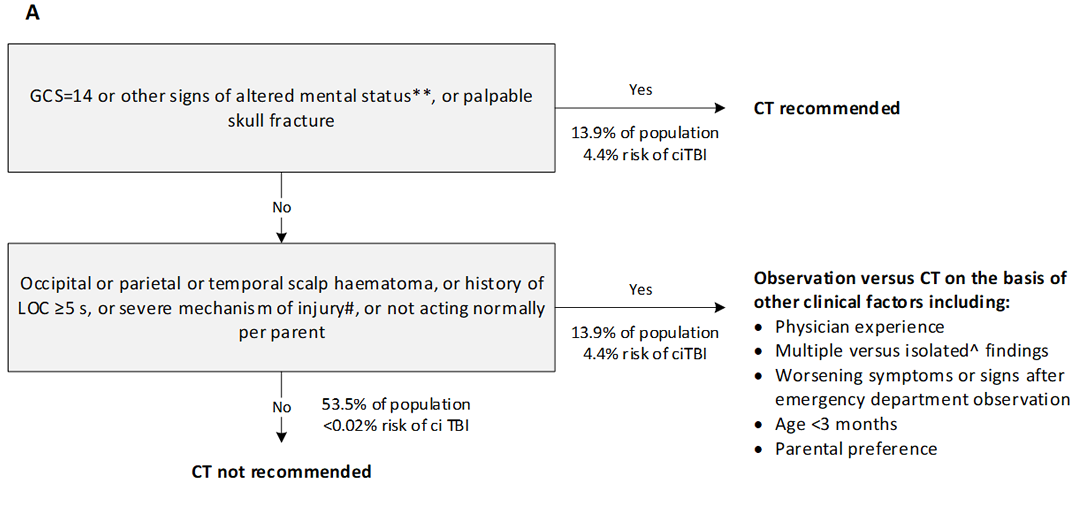
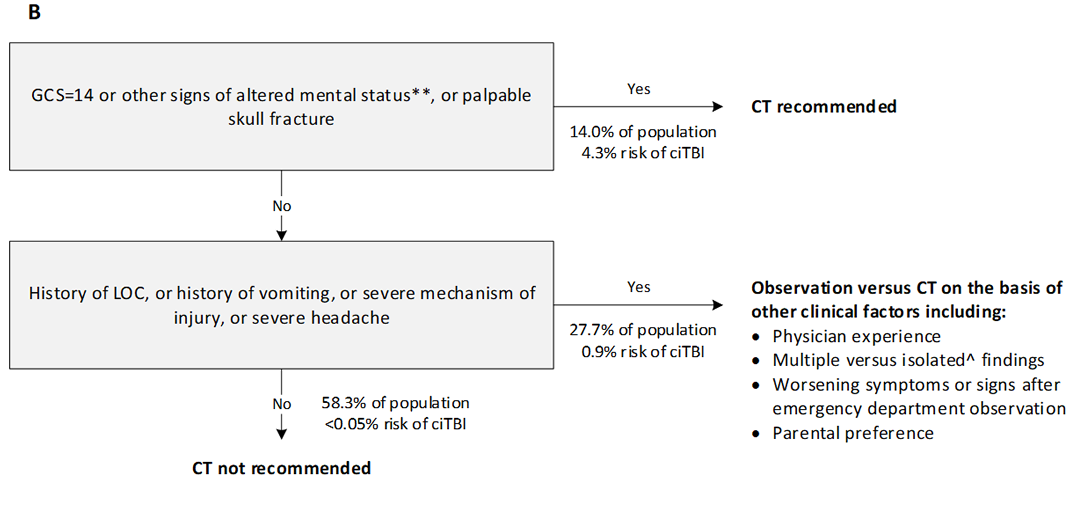
GCS=Glasgow Coma scale. ciTBI clinically-important traumatic brain injury. LOC=loss of consciousness. *Data are from the combined derivation and validation populations. **Other signs of altered mental status: agitation, somnolence, repetitive questioning, or slow response to verbal communication. #Severe mechanism of injury: motor vehicle crash with patient ejection, death of another passenger, or rollover; pedestrian or bicyclist without a helmet struck by a motorised vehicle; falls of more than 0.9 m (3 feet) (or more than 1.5 m [5 feet] for panel B); or head struck by a high-impact object. ^Patients with certain isolated findings (ie, with no other findings suggestive of traumatic brain injury), such as isolated LOC, isolated headache, isolated vomiting, and certain types of isolated scalp haematomas in infants older than 3 months, have a risk of ciTBI substantially lower than 1%. Risk of ciTBI exceedingly low, generally lower than risk of CT-induced malignancies. Therefore, CT scans are not indicated for most patients in this group.
Reproduced from Kuppermann N, Holmes, J, Dayan P. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. The Lancet 2009; 374(9696):1160-70 with permission from Elsevier Ltd. Link to article.
The children’s head injury algorithm for the prediction of important clinical events rule (CHALICE)
| History | Examination | Mechanism |
|---|---|---|
|
|
|
If none of the above variables are present, the patient is at low risk of intracranial pathology. | ||
*Equivalent to over 64km/hour
Reproduced from Dunning J, Daly JP, Lomas J, et al. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Archives of Disease in Childhood 2006; 91:885-891 with permission from BMJ Publishing Group Ltd. Link to article
Assessment
Emergency care should always involve a rapid primary survey with evaluation of (and immediate management of concerns with) airway, breathing, circulation and disability (ABCD). This includes level of consciousness and Glasgow coma score (GCS).
The aim of the assessment is to:
- identify a child with a severe head injury at risk or showing signs of raised intracranial pressure (ICP) to enable immediate investigation, management and prompt referral
- differentiate children at low risk of a clinically significant head injury (who can be safely discharged without the need for a CT scan) from those who require further management (CT scan or observation).
- identify children with other concerns e.g. non-accidental injury (NAI), alternate diagnoses.
An integration of clinical assessment features into low, intermediate and high risk is included in head injury management (see table on risk stratification in Management section).
History and mechanism of injury
Important factors to elicit on history include:
- abnormal behaviour such as agitation or drowsiness
- loss of consciousness
- vomiting
- post traumatic seizure
- amnesia: retrograde and antegrade
- headache
- mechanism of injury – significant mechanisms of injury include:
- fall from a significant height
- motor vehicle accident (especially if high-speed, ejected from vehicle or others significantly injured in the same crash)
- pedestrian/cyclist impacted by a motor vehicle
- impact from high-speed projectile e.g. golf ball, ceiling fan
- any special circumstances to consider including injuries of potential NAI concern and alternate diagnoses (refer to Further Assessment Considerations below)
Some differences exist between CDRs in symptoms and signs included as important, and the degree e.g. height of fall, number of vomits, length of loss of consciousness.
Examination
Following the primary survey, a thorough head-to-toe examination (secondary survey) with specific attention paid to the maintenance of neutral positioning if cervical spine injury concerns (refer to Cervical spine injury guideline) should occur.
Particular features on examination to identify are:
- level of consciousness (LOC), including GCS or AVPU score. All CDRs consider that a child with a diminished or decreasing LOC is at significant risk of intracranial injury. If present, examination should be made for other signs of raised intracranial pressure (ICP)
- suspicion of an open or depressed skull fracture (including boggy haematomas, palpable depressions)
- signs of a basal skull fracture (raccoon eyes, haemotympanum, Battle’s sign, CSF leak via nose, ears)
- penetrating injury
- presence of focal neurological deficit
- other features suggestive of more extensive injury (e.g. other significant trauma, NAI)
- in infants and young children: size and location of a haematoma, swelling or laceration should be noted, as should a bulging fontanelle (see PECARN and CHALICE CDRs)
Signs of raised ICP
- deteriorating or diminished LOC
- abnormal posture (decorticate or decerebrate)
- abnormal pupillary responses, unilateral or bilateral dilatation
- abnormal oculocephalic reflexes (doll’s eye movement or dysconjugate upward gaze)
- abnormal breathing patterns (hyperventilation, Cheyne-Stokes, apnoea)
Cushing’s triad (hypertension + bradycardia + breathing abnormalities) is a late sign.
Careful initial and repeated clinical examination is required to identify signs of raised ICP.
Seek urgent paediatric critical care/neurosurgical advice for a child with signs of raised ICP or decreased level of consciousness (onsite or via Retrieval Services Queensland (RSQ).
Further assessment considerations
Consider special circumstances in the presentation, or the possibility that the clinical presentation is unrelated to the head injury.
| Further assessment considerations in children presenting with head injury | |
|---|---|
| Non-accidental Injury (NAI) | Concerns of NAI necessitate mandatory discussion with senior emergency clinicians/paediatricians. Injuries of concern may include those where the extent of injury is inconsistent with the mechanism provided e.g. inadequate explanation for a skull fracture in an immobile child. Further investigation may be required. |
| Multi-trauma patients | Consider other injuries and impact on physiology. |
| Cervical spine injury | Maintain precautions and consider further imaging if concerns exist. |
| Patient-specific risk factors | Consider other factors which may increase risk of intracranial injury independent of mechanism e.g. coagulopathy. |
| Non-mechanical falls | Consider further investigation if head injury associated with a non-mechanical fall e.g. ECG. |
| Alternate diagnoses | Consider alternative explanations for the presenting picture (e.g. LOC, vomiting). Metabolic conditions, infectious diseases, poisoning, acute surgical conditions and nonconvulsive status may present with similar symptoms i.e. the head injury may not be the cause of the symptoms. |
Investigations
CT scan remains the gold standard investigation of intracranial injury in the acute setting. Scanning is not recommended in all children due to the associated radiation risks (dependent on the machine and site-specific protocols), the need for sedation and/or transfer and resource costs.
Pharmacological sedation,7 if required, should be performed by senior medical staff experienced with the agents used and airway management in children. Small doses of Midazolam (intravenous / nasal / buccal) or intravenous Ketamine are often used. Anaesthetic assistance may be required.
Very young infants may settle sufficiently with swaddling / wrapping after a feed if the clinical situation permits. Oral sucrose may facilitate comfort during the scan.
Management according to risk stratification
Refer to flowchart [PDF 342.68 KB] for a summary of the emergency management in children who present with a head injury
Risk stratification heavily informs the management of the head injured child. CDRs (PECARN, CHALICE) may be used to assess this risk, accepting that strict application of these rules in our setting is likely to significantly increase baseline imaging rates with no appreciable increase in identification of significant intracranial injury.5 The following approach is proposed to guide imaging, observation and discharge decisions, incorporating the CDRs, and allowing for clinical judgement.
| Low risk ALL of the following: | Intermediate risk No high-risk features and ≥1 of the following: | High-risk ≥1 of the following: |
|---|---|---|
|
|
|
Low-risk children
Children may be considered at “low risk” if they have all of the following:
- history of head trauma with no concerning features on history, examination or mechanism of injury (i.e. no risk factors for intermediate or high-risk head injury)
AND - normal level of consciousness (GCS 15).
As per evidence available from published decision rules,2,3 these children are considered to be at very low risk of having a clinically significant head injury (<0.1%) and may be discharged home with head injury advice if other discharge criteria are met (see Disposition).
Low risk/minor head injury is not no risk.
All carers of children discharged, whether or not imaging has been performed, should receive verbal and written head injury advice.
Post-concussive symptoms and adverse neuropsychological sequalae can occur following a minor head injury.8,9 Carers should always be advised to seek medical attention if low grade or vague symptoms persist. Return to sport advice, if applicable, should also be provided.
Intermediate-risk children
Seek senior emergency/paediatric advice as per local practice prior to requesting a CT scan for a child at intermediate risk of an intracranial injury.
Seek urgent paediatric neurosurgical advice (onsite or via RSQ) if abnormalities are identified on CT scan.
Seek paediatric neurosurgical/local paediatric advice as per local practice for a child with significant persistent symptoms and no abnormality detected on CT scan.
Intermediate risk patients include those with a GCS 14 – 15 but concerning features on history, examination or mechanism of injury. Children who have any concerning features are at increased risk of a clinically significant head injury compared to those who do not, and further investigation or observation should be considered.
While CT scan is the gold standard investigation to exclude clinically significant head injuries in the acute situation, scanning all children who have concerning features is likely to result in an unacceptably high rate of CT use in our population, and may necessitate transfer. As such, a period of observation may be an acceptable alternative in some situations. This decision should be made in consultation with senior medical staff and can only occur where appropriate facilities and experienced staff are available to monitor the child during the period of observation with timely intervention / investigation if required.
Factors that may influence this decision include:
- clinician experience
- presence of multiple risk factors
- worsening or unresolved symptoms
- age of the child (need for sedation in younger children)
- availability of local resources for imaging and where relevant, sedation
Examples of situations that may be appropriate for observation include an otherwise well child subjected to a significant mechanism of injury; or a child with a history of isolated infrequent vomiting who appears completely well.
The optimal time for observation is unclear. Most guidelines, including NICE, recommend a minimum period of at least four hours from the time of injury.10 A large Canadian retrospective review found that most children with a significant intracranial injury are symptomatic within six hours of injury.11
Observation
Children at an intermediate risk of an intracranial injury undergoing observation should be closely monitored for signs of deterioration.
For a child with a GCS of 14 and no high-risk features, half hourly observations are recommended until the GCS is 15.9
| Time post-injury | Frequency |
|---|---|
| Up to 2 hours | Half-hourly |
| 2-6 hours | Hourly |
| ≥6 hours | 2-hourly |
Seek senior emergency/paediatric advice as per local practice if symptoms persist, worsen or progress within the observation period. A CT scan is recommended.
Seek urgent paediatric critical care/neurosurgical advice (onsite or via RSQ) if significant clinical deterioration occurs within the observation period. Emergency management may be required.
High-risk children and those with life-threatening injuries
Seek urgent paediatric critical care/neurosurgical advice (onsite or via RSQ) for a child with life-threatening or severe head injuries. Emergency craniotomy may be required.
Indications for immediate CT scan with high-risk patients include:
- GCS <14
- suspicion of a depressed, open or basal skull fracture
- penetrating injury
- NAI concerns
- presence of focal neurological deficit
In infants and young children, the size or location of a haematoma, swelling or laceration (suspicious for skull fracture) or a bulging fontanelle may also warrant consideration of immediate CT scan.
Concurrent investigation, management and referral may be required for the child or infant presenting with a high-risk of a significant intracranial injury. Priorities include:
- ABC assessment and management
- active management of raised ICP if suspected
- consideration of other serious injuries
- frequent clinical reassessment to examine for signs of deterioration
- urgent CT scan if available OR urgent transfer if required
- consideration of early liaison with neurosurgical and critical care services (onsite or via RSQ)
Immediate management of raised ICP
Both generalised cerebral oedema and focal haemorrhage / swelling may produce raised ICP in children. Management aims to prevent further rises in ICP and/or remove its cause (surgical evacuation of haematoma) whilst maintaining adequate cerebral perfusion.
| Immediate management of raised ICP | |
|---|---|
| Airway and breathing |
|
| Circulatory support |
|
| Head tilt |
|
| Hyperosmolar agents |
|
| Sodium Chloride 3% (IV) dosing for the treatment of raised ICP | |
|---|---|
| Sodium Chloride 3% (Hypertonic Saline 3%) (IV) |
3 mL/kg/dose (1–5 mL/kg/dose) over 10-15 minutes 3 mL/kg is expected to increase plasma sodium by approximately 2-3 mmol/L |
| Risks |
Rebound ICP Central pontine myelinosis Subarachnoid haemorrhage Renal failure |
| Mannitol (IV) dosing for the treatment of raised ICP | |
|---|---|
| Mannitol (IV) |
0.25-0.5 g/kg over 10-15 minutes Higher doses i.e. 1 g/kg may be administered on senior advice. |
| Risks |
Hypotension Hyperosmolality Rebound elevations in ICP Renal failure Extravasation |
Other measures in ICP management
- actively manage seizures according to Status epilepticus guideline. Post-traumatic seizure management may include use of second-line agents for stabilisation and avoidance of further seizures (e.g. Levetiracetam; Phenytoin); seek critical care advice. Current evidence does not support prophylaxis with second-line agents if a seizure has not occurred.
- provide adequate analgesia and sedation
- consider neuromuscular blockade (note that paralysis may mask seizure activity)
- avoid hyperthermia
Further considerations in head injury management
Pain management
- appropriate attention should be given to pain relief.
Anti-emetic therapy
Control of nausea and vomiting following a head injury with anti-emetic use (Ondansetron) should be strongly considered when the decision to CT scan has already been made. Its use prior to this decision remains under some debate, although use is increasing. Two US retrospective studies 25,26 found that its use was not associated with an increase in missed diagnoses, however, both were not powered to definitively make this conclusion and the use of Ondansetron in children not scanned was very low (2%). One study found an increased representation rate with use.25
A secondary analysis of both PECARN and APHIRST studies, showed clinically significant intracranial injury was very uncommon when isolated vomiting was the only risk factor present, and that observation rather than immediate CT appears to be an appropriate management strategy in these children.27-28 Careful history and examination should be undertaken to ensure that vomiting is truly an isolated risk factor, with consideration for close observation where required. <
| Ondansetron for the management of vomiting in children | |
|---|---|
| Dose (Oral or IV) |
0.15 mg/kg (maximum 8 mg).
Wafers and oral dissolvable tablets are available in 4 mg and 8 mg doses. If using either of these the recommended doses are as follows:
Not recommended if aged less than 6 months, weight less than 8 kg or with ileus. A single dose may be sufficient. Repeat at eight-hourly intervals if required. |
| Considerations | Ondansetron prolongs the QT interval in a dose –dependent manner. Exercise caution in children who have or may develop prolongation of QTc (e.g. those with electrolyte disturbances, heart failure or on medications that may lead to a prolongation of the QTc). |
Escalation and advice outside of ED
Clinicians can contact the services below if escalation of care outside of senior clinicians within the ED is needed, as per local practices. Transfer is recommended if the child requires a higher level of care.
Critically unwell or rapidly deteriorating child
| Includes children with the following (as a guide): | |||
|---|---|---|---|
| |||
| Less than 2 weeks | Less than 1 year | 1-8 years | Over 12 years |
|
|
|
|
| Reason for contact | Who to contact |
|---|---|
| For immediate onsite assistance including airway management | The most senior resources available onsite at the time as per local practices. Options may include:
|
| Paediatric critical care advice and assistance | Onsite or via Retrieval Services Queensland (RSQ). If no onsite paediatric critical care service contact RSQ on 1300 799 127:
RSQ (access via QH intranet) Notify early of child potentially requiring transfer. Consider early involvement of local paediatric/critical care service. In the event of retrieval, inform your local paediatric service. |
Non-critical child
| Reason for contact by clinician | Who to contact |
|---|---|
| For specialist advice on management, disposition and follow-up of a child with an abnormality identified on CT scan. | Onsite/local paediatric neurosurgical service as per local practice. If no onsite/local paediatric neurosurgical service, seek advice via Children’s Advice and Transport Coordination Hub (CATCH) on 13 CATCH (13 22 82) |
| For specialist advice on management, disposition and follow-up of children who have persistent symptoms despite normal imaging. | Onsite/local paediatric neurosurgical service as per local practice. If no onsite/local paediatric neurosurgical service, seek advice via Children’s Advice and Transport Coordination Hub (CATCH) on 13 CATCH (13 22 82) or contact the onsite/local paediatric service as per local practice. |
| For advice regarding NAI concerns. | Onsite/local paediatric service as per local practice. |
Inter-hospital transfers
| Do I need a critical transfer? |
|
| Request a non-critical inter-hospital transfer |
|
| Non-critical transfer forms |
|
Disposition
When to consider discharge
A child may be safely discharged after a head injury if ALL of the following criteria are met:
- one of the following:
- low risk, or intermediate risk with unremarkable period of observation
- negative CT Scan and no significant persistent symptoms / signs
- well child with cranial injury deemed suitable for discharge following neurosurgical review e.g. some non-depressed linear skull fractures without intracranial injury.
- GCS remains at 15
- no NAI concerns
- no concerns of serious alternate / concurrent diagnosis
- parental / carer concerns adequately addressed
- parent/carer can safely manage the child at home and can return in the event of deterioration. Time of day, language barriers and other demands on a caregiver’s time should be considered.
Written and verbal information should be provided to parents/carers on discharge, including possible post concussive symptoms syndrome. Advice on return to sport should also be provided where indicated. Parents should be advised to seek medical review if any concerns arise. See Head injury factsheet.
When to consider admission
Admission to an inpatient service and/or transfer is advised if children fail to meet discharge criteria; neuroimaging, if not already undertaken may be indicated.
Related documents
Guidelines
Factsheet
-
- Acworth, J., Bab, l.F., Borland, M., Ngo, P., Krieser, D., Schutz, J. et al. (2009), ‘Patterns of presentation to the Australian and New Zealand Paediatric Emergency Research Network’, Emerg Med Australas, Feb; 21(1): pp. 59-66.
- Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Jr., Atabaki SM, Holubkov R, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160-70.
- Dunning J, Daly JP, Lomas JP, Lecky F, Batchelor J, Mackway-Jones K, et al. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child. 2006;91(11):885-91.
- Osmond MH, Klassen TP, Wells GA, Correll R, Jarvis A, Joubert G, et al. CATCH: a clinical decision rule for the use of computed tomography in children with minor head injury. CMAJ. 2010;182(4):341-8.
- Babl FE, Borland ML, Phillips N, Kochar A, Dalton S, McCaskill M, et al. Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet. 2017;389(10087):2393-402.
- Babl FE, Oakley E, Dalziel SR, Borland ML, Phillips N, Kochar A, et al. Accuracy of Clinician Practice Compared With Three Head Injury Decision Rules in Children: A Prospective Cohort Study. Annals of emergency medicine. 2018.
- Starkey, E., Sammons, H.M. (2011), ‘Sedation for radiological imaging’, Arch Dis Child Educ Pract Ed., Jun;96(3): pp.101-6.
- Sroufe, N.S., Fuller, D.S., West, B.T., Singal, B.M., Warschausky, S.A., Maio, R.F. (2010), ‘Postconcussive symptoms and neurocognitive function after mild traumatic brain injury in children’, Pediatrics, Jun: 25(6): pp. e1331-9.
- Babcock-Cimpello, L., Blyth, B., Bazarian, J.J. (2004), ‘Decision rules for computed tomographic scans in children after head trauma’, Ann Emerg Med., Jul;44(1): pp. 90-1; author reply 1-2.
- National Institute for Health and Clinical Excellence. (2017), Head injury: assessment and early management of head injury CG 176, National Institute for Health and Clinical Excellence: London.
- Hamilton, M., Mrazik, M., Johnson, D.W. (2010), ‘Incidence of delayed intracranial hemorrhage in children after uncomplicated minor head injuries’, Pediatrics, Jul;126(1): pp. e33-9.
- Su, F., Raghupath, R., Huh, J. (2010), Neurointensive Care for Traumatic Brain Injury in Children, [online], [cited 16/07/2010].
- Adelson, P.D., Bratton, S.L., Carney, N.A., Chesnut, R.M., du Coudray, H.E., Goldstein, B. et al. (2003), ‘Chapter 12. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury’, in ‘Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents‘, in Pediatr Crit Care Med. Jul; 4(3 Suppl): pp. S45-8.
- Roosevelt, G., Paradis, N. (2008), ‘Cerebral resuscitation’, in Baren, J., Rothrock, S., Brennan, J., Brown, L. (eds), Pediatric Emergency Medicine, Elsevier: Philadelphia; pp. 94-105.
- Filanovsky, Y., Miller, P., Kao, J. (2010), ‘Myth: Ketamine should not be used as an induction agent for intubation in patients with head injury’, CJEM, Mar;12(2): pp.154-7.
- Himmelseher, S., Durieux, M.E. (2005), ‘Revising a dogma: ketamine for patients with neurological injury?’, Anesth Analg., Aug;101(2): pp.524-34, (table of contents).
- Sehdev, R.S., Symmons, D.A., Kindl, K. (2006), ‘Ketamine for rapid sequence induction in patients with head injury in the emergency department’, Emerg Med Australas., Feb;18(1): pp. 37-44.
- Gwer, S., Gatakaa, H., Mwai, L., Idro, R., Newton, C.R. (2010), ‘The role for osmotic agents in children with acute encephalopathies: a systematic review’, BMC Pediatr., 10(1): pp. 23.
- Kamel, H., Navi, B.B., Nakagawa, K., Hemphill, J.C. 3rd, Ko, N.U. (2011), ‘Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials’, Crit Care Med. Mar;39(3): pp.554-9.
- Wakai, A., Roberts, I., Schierhout, G. (2005), ‘Mannitol for acute traumatic brain injury’, cassette recording, Cochrane Database Syst Rev., (4): CD001049, [online]
- Tavakkoli, F. (2011), ‘Review of the role of mannitol in the therapy of children’, Geneva: World Health Organisation.
- Roumeliotis N, Dong C, Pettersen G, Crevier L, Emeriaud G. Hyperosmolar therapy in pediatric traumatic brain injury: a retrospective study. Childs Nerv Syst. 2016;32(12):2363-8
- Burgess S, Abu-Laban RB, Slavik RS, Vu EN, Zed PJ. A Systematic Review of Randomized Controlled Trials Comparing Hypertonic Sodium Solutions and Mannitol for Traumatic Brain Injury: Implications for Emergency Department Management. The Annals of pharmacotherapy. 2016;50(4):291-300.
- Kumar SA, Devi BI, Reddy M, Shukla D. Comparison of equiosmolar dose of hyperosmolar agents in reducing intracranial pressure-a randomized control study in pediatric traumatic brain injury. Childs Nerv Syst. 2019;35(6):999-1005.
- Green-Hopkins I, Monuteaux MC, Lee L, Nigrovic L, Mannix R, Schutzman S. Use of Ondansetron for Vomiting After Head Trauma: Does It Mask Clinically Significant Traumatic Brain Injury? Pediatr Emerg Care. 2017.
- Sturm J, K. Simon H, Khan N, A. Hirsh D. The use of ondansetron for nausea and vomiting after head injury and its effect on return rates from the pediatric ED. Am J Emerg Med. 166–72
- Dayan PS, Holmes JF, Atabaki S, Hoyle J, Jr., Tunik MG, Lichenstein R, et al. Association of traumatic brain injuries with vomiting in children with blunt head trauma. Ann Emerg Med. 2014;63(6):657-65.
- Borland ML, Dalziel SR, Phillips N, Dalton S, Lyttle MD, Bressan S, et al. Vomiting With Head Trauma and Risk of Traumatic Brain Injury. Pediatrics. 2018;141(4).
-
Document ID: CHQ-GDL-60023
Version number: 3.0
Supersedes: 2.0
Approval date: 11/12/2020
Effective date: 11/12/2020
Review date: 11/12/2023
Executive sponsor: Executive Director Medical Services
Author/custodian: Queensland Emergency Care Children Working Group
Applicable to: Queensland Health medical and nursing staff
Document source: Internal (QHEPS) + External
Authorisation: Executive Director Clinical Services
Keywords: Paediatric, emergency, guideline, head injury, intracranial, PECARN, CHALICE, 60023
Accreditation references: NSQHS Standards (1-8): 1 Clinical Governance, 4 Medication Safety, 8 Recognising and Responding to Acute Deterioration
ISO 9001:2015 Quality Management Systems: (4-10) -
This guideline is intended as a guide and provided for information purposes only. View full disclaimer.
Last updated: March 2024